The EPLO’s Intensive course “Biolaw and Biotechnology: The crossroads of Science and Regulation” is designed to combine an accurate description of cutting-edge applications in Biomedicine and Biotechnology with the knowledge of essential elements related to ethics and legal regulation. The program is conducted in English, in collaboration with the EPLO Institute for Sustainable Development.
The course, taking into account the post-COVID era’s challenges, aims at giving answers to questions such as
– Can modern Biotechnology support a growth based on the efficient use of resources by moving to a clean, circular economy in the Green Deal context?
– Can modern Biotechnology improve the public health with novel vaccines and pharmaceuticals in the frame of precision medicine, and guaranty a healthy food supply?
– Can we “do things with rules”? Is the existing legal framework appropriate for ensuring a balance between the impressive potential of the Bioeconomy and the protection of key values, such as environmental sustainability and public health? How do we measure and assess their impact on the environment, on social issues and on governance?
The program is interdisciplinary, combining knowledge from heterogeneous scientific fields, such as biology, law, medicine and engineering.
Course Description
The European Green Deal is the EU’s plan to make the EU’s economy sustainable at all economic sectors and vertical markets. This can be done by turning climate and environmental challenges, the most pressing issues of our times, into opportunities, and making the transition to a sustainable future just and inclusive for all, while accomplishing net-zero objectives and the Agenda 2030 SDGs.
The EU Green Deal as a vital European vision for the 21st century and the European economy is closely dependent on the rapidly growing sector of Bioeconomy, an area that comprises several economic sectors, academic disciplines, and areas of policy. It encompasses the production of renewable biological resources and the conversion of these resources and waste streams into value-added products such as food, feed, bio-based products, and bioenergy.
Over the last three decades, Bioeconomy mobilizes Biomedicine and Biotechnology’s novel applications in a vast range of productive activities worldwide, from Agriculture, Food production, and Energy to Pharmaceuticals and new medical diagnostic tools and therapeutic means.
The challenges that emerged for the Environment and Public Health due to the COVID-19 pandemic have revolutionized the way we do research in the bio sciences and how we find marketable applications in our direct future; the post-COVID era will inevitably be the era of a great boost in novel technologies, starting from the recent examples of vaccines’ development.
Yet, this exciting potential cannot be understood without referring to its regulatory dimension. Regulation in that area remains largely unknown, although biological innovation has triggered intensive debates of policymakers regarding bioethics and biolaw, resulting in a significant amount of detailed rules at the international, EU, and national levels. Nowadays, it seems impossible for professionals active in the broad spectrum of the Bioeconomy as it encompasses the production of renewable biological resources and their conversion to value-added products such as food, feed, bio-based products, and bioenergy to undertake initiatives involving novel technologies without considering the regulatory context in relevance.
Who is it for?
- Lawyers specialized in medical or environmental consulting and litigation;
- Medical doctors, health professionals;
- Research professionals;
- Managers, advisors in Pharma, Food, Energy, Health, Agricultural industry;
- Members and employees in environmental and medical NGOs;
- Civil servants and state managers involved in the designing and implementation of regulation relevant to biomedicine and biotechnology;
- Students (Law, Medicine, Biology, Sociology, Political Sciences).
Instructors (as per program schedule)
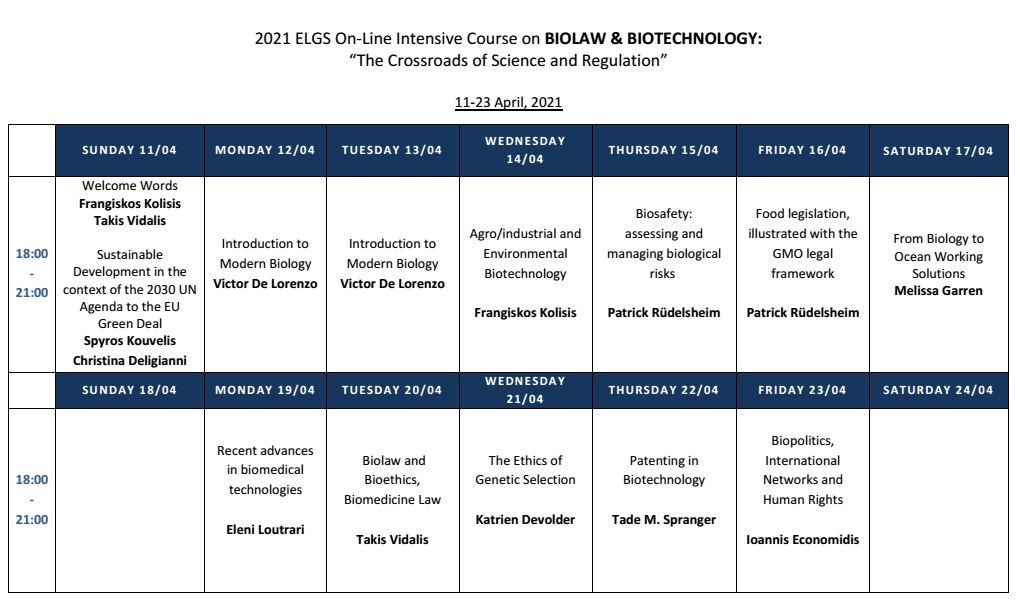
11/4/2021, 18.00 – 18.10
Welcome:
Prof. F. Kolisis & Dr. P. Vidalis
18.10 – 20.00
Spyros Kouvelis, Director, Institute for Sustainable Development, EPLO/Senior Associate, Cambridge Institute for Sustainability Leadership, University of Cambridge
Christina Deligianni, Public Policy Officer, Institute for Sustainable Development, EPLO, “Introduction to Sustainable Development”
12 & 13/4/2021, 18.00 – 21.00
Prof. Victor De Lorenzo, Systems Biology Department, Centro Nacional de Biotecnologia, Madrid. “Introduction to modern biology” ( Synthetic Biology – Reshaping the gene expression flow – Editing and rewriting genomes -From the test tube to planet Earth)
14/4/2021, 18.00 – 21.00
Prof. Frangiskos Kolisis, Chemical Engineering Department, National Technical University of Athens. “Agro/industrial and Environmental Biotechnology”
15 & 16 /4/2021, 18.00 – 21.00
Dr. Patrick Rüdelsheim, General Manager, Perseus Biosafety Company, Belgium. “ Biosafety: assessing and managing biological risks” and “Food legislation, illustrated with the GMO legal framework”
17/4/2021, 18.00 – 20.00
Business case:
Dr Melissa Garren, Founder and CEO, WorkingOcean “From Biology to Ocean Working Solutions”
19/4/2021, 18.00 – 21.00
Dr. Heleni Loutrari, Medical School, National Kapodestrian University of Athens. “Recent advances in biomedical technologies”
20/4/2021, 18.00 – 21.00
Dr. P. Vidalis, Hellenic Republic, National Bioethics Commission, ”Biolaw and Bioethics, Biomedicine Law”
21/4/2021, 18.00 – 20.00
Dr Katrien Devolder, Senior Researcher, Oxford Uehiro Centre for Practical Ethics, Oxford University. “The Ethics of Genetic Selection”
22/4/2021, 18.00 – 20.00
Prof. Tade M. Spranger, Professor of Law, Institute of Public Law, University of Bonn
“Patenting in Biotechnology”
23/4/2021, 18.00 – 21.00.
Dr. Ioannis Economidis, ex. Scientific officer of European Commission, Brussels, “Biopolitics, International Networks and Human Rights”
Program Structure
Learning Outcome?
Participants should be able to:
• Demonstrate knowledge and understanding of the basic concepts of sustainable development, the EU Green Deal, biotechnology trends, and bioeconomy.
• Understand the vital role of regulation in relevant policies, with reference to the principles of contemporary biolaw and bioethics.
• Demonstrate a familiarity with the relevant literature and the leading debates addressing pressing issues.
• Be able to formulate opinions with specific documentation in the above described areas and participate in the emerging discourse at the European and global levels.